Progress in the high-temperature corrosion mechanism of carburized 316H stainless steel in
molten chloride salts for bearings applied in next-generation concentrated solar power plant
Recently, the alloy research team of Shanghai Institute of Applied Physics (SINAP), Chinese Academy of Sciences (CAS) made progress in the carburized 316H stainless steel in molten chloride salts and proposed a corrosion mechanism of carbides in high-temperature molten salts, testing the possibility of the application of carburized 316H as bearings materials in the molten salt pump. The research result entitled “Corrosion behavior of carburized 316 stainless steel in molten chloride salts” was published in Solar Energy, which is the official journal of the International Solar Energy Society. The co-first authors are postgraduate student Sen Ren and Researcher Associate Yanjun Chen, and the co-corresponding authors are Prof. Xiang-Xi Ye, Prof. Jianping Liang, and Associate Prof. ZeZhong Chen (from the University of Shanghai for Science and Technology) .
Compared with the traditional concentrated solar power plants (CSP, operating temperature 565 ℃), the next-generation CSP uses chloride salts as the thermal energy storage and heat transfer media, whose operating temperature can reach above 700 ℃ to achieve higher energy conversion efficiency. To date, most high temperature pumps for molten salts are of the short-shaft cantilever type to avoid undesirable vibration, since no bearing materials had yet been demonstrated to work in hot molten salts (above 565 ℃), which has been one of the bottlenecks of the long-shaft molten salt pump for a long time. Because the molten salts intrinsically are highly corrosive, an essential requirement of materials for pump bearings in contact with molten salts at high temperatures is excellent corrosion resistance to molten salts. In addition, the materials should resist wear, galling, and self-welding under severe operating conditions. As an important candidate alloy for CSP, 316H stainless steel has good high temperature mechanical properties and economy, but its hardness is only 180HV and its friction and wear resistance is poor. Carburization is a common technique for enhancing the wear resistance of an alloy, primarily by increasing the number of carbides on the alloy surface, which increases the alloy's surface hardness and thus its wear resistance. However, it is still controversial whether carbides can affect the corrosion behavior of alloys in molten salts. The study of Oak Ridge National Laboratory (ORNL) on carburized Hastelloy N alloy and our recent study on decarburized GH3535 alloy (Acta Metall.Sin.-Engl.,32 (2019) 401-412) showed that the number of surface carbides has little effect on the corrosion degree of UNS N10003 alloy. However, our study (Corros.Sci.,133 (2018)) and the study of University of Wisconsin (J. Fluorine Chem.130 (2009) 67-73.) also found that carbides in 800H alloys can accelerate the corrosion of alloys in molten salts. To verify the feasibility of carburized 316H as bearing materials, we first explored the carburizing process parameters of 316H stainless steel, and the surface hardness of the obtained carburized 316H is higher than 550 HV by vacuum carburization and aging treatment. Synchrotron radiation XRD results show that the surface and near-surface grain boundary carbides are mainly Cr7C3. Then the corrosion of carburized 316H and uncarburized 316H, was evaluated in molten chloride salts (NaCl-KCl-MgCl2) with Mg addition at 700 ℃. The results showed that carburized 316H has severe intergranular corrosion (80 μ m), while the uncarburized one has little corrosion (less than 10 μ m). The surface hardness of carburized 316H decreases to 400HV after corrosion. Through the characterization of corrosion cross section morphology and composition, it is found that Cr7C3 carbide in carburized 316H is easily corroded by impurities (hydrate, metal ions) of molten chloride salt. The impurities in the molten salt can easily react with Cr on the alloy surface, and the Cr content of the Cr7C3 carbide on the surface and grain boundary is more than twice that of the Cr of the alloy matrix, which creates a great concentration gradient of Cr between the Cr7C3 carbide and the alloy matrix. Additionally, the diffusion rate of Cr along the grain boundary is much faster than that in the alloy matrix. Therefore, Cr in the Cr7C3 carbide is preferentially corroded at the surface and grain boundary by the impurities of the molten chloride salts, resulting in severe intergranular corrosion. The depletion of Cr from Cr7C3 carbide causes the decomposition of the Cr7C3 carbides, reducing the hardness of the carburized 316H. The annual corrosion rates of 316H and carburized 316H in the chloride salts with Mg addition were estimated to be 47 μm and 328 μm, respectively, based on the corrosion diffusion rate of Cr. Because the annual corrosion rate of alloy for CSP is suggested less than 50 μm, it may be deduced that 316H can be compatible with the molten chloride salts with Mg addition, and impurity in the chloride salts should be strictly limited for the application of carburized 316SS in the molten salt environment.
The research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences and the Youth Innovation Promotion Association, CAS.
Link:https://doi.org/10.1016/j.solener.2021.05.057
Fig. 1 Cross-sectional SEM images of the corroded alloy samples in molten NaCl-KCl-MgCl2 salts at 700 ?C for 400 h. (a) 316H surface; (b) carburized 316H surface.
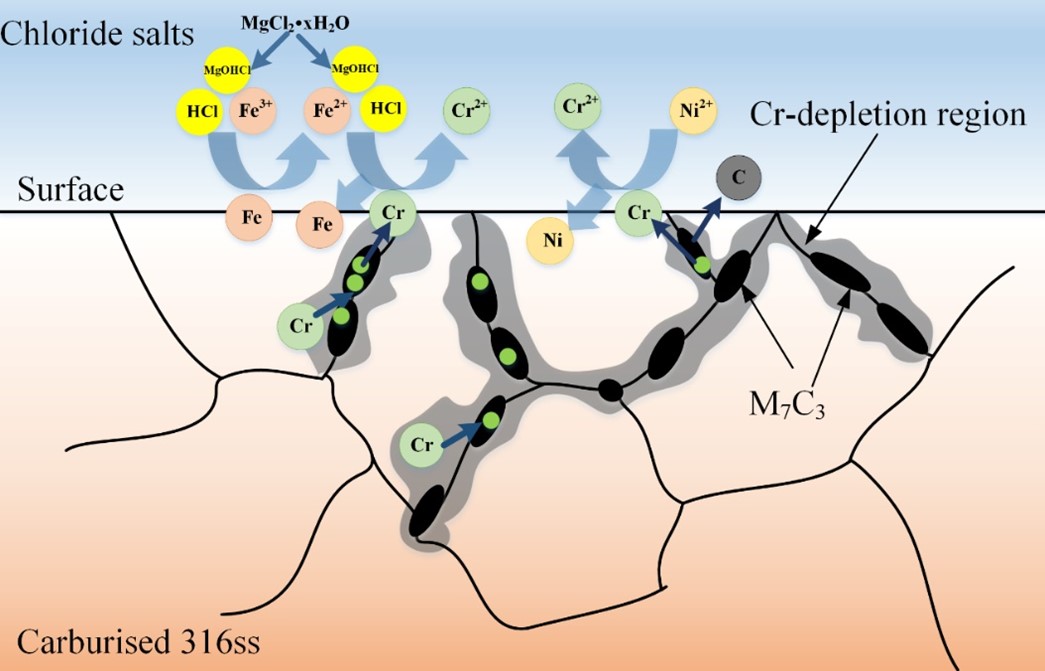
Fig. 2. Schematic depiction of the corrosion of carburized 316H in molten chloride salts.








