






As excellent thermal energy storage and heat transfer medium, molten salts have broad application potential in the new generation energy system, such as molten salt reactor and concentrating solar power. Because molten salts are highly corrosive to metallic structural materials, the development of new alloys resistant to high temperature molten salt has become an important issue in the molten salt application, which has received attention in recent years from the United States, the European Union, and other national organizations. Previous researches have shown that fluoride-forming alloying elements such as Cr, Fe, and Al are easily attacked by oxidizing impurities (H2O, O2, Ni, Fe, and other metallic ions) in molten salts, and the corrosion degree of the alloy is determined by the diffusion rate of Cr in the alloy matrix (the depth of Cr-depletion layer). Therefore, the contents of Cr, Fe and Al in the alloys for molten salt application are strictly limited, only a small amount of which is added to balance the high temperature oxidation resistance. Some related alloy design criteria that control the total content of elements easily corroded by molten salt have been proposed and applied. However, these criteria mainly consider the impurity-driven corrosion in molten salt based on Gibbs free energy (first-order effect), and ignore the corrosion-induced chemical interactions between alloying elements (second-order effect).
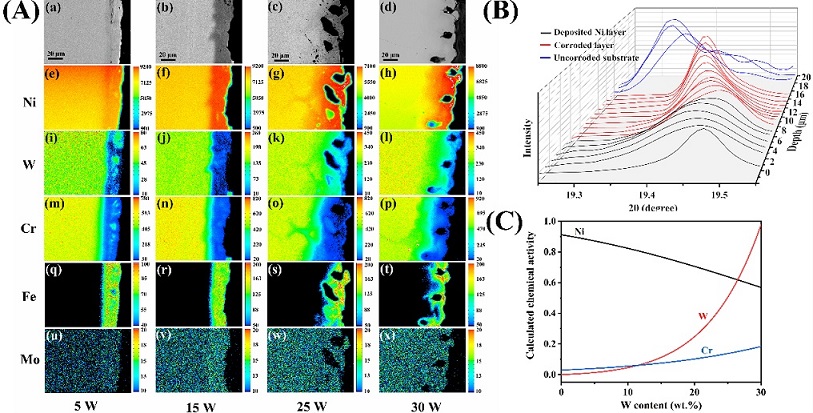
GH3539 (Chinese alloy designation, Ni-(26-28)wt.%W-6wt.%Cr alloy), developed by SINAP, have been considered one of the potential advanced structural materials for molten fluoride salt techniques. Previous studies showed that Ni-26W-6Cr alloy possesses a great corrosion resistance to purified molten FLiNaK salt (46.5LiF-11.5NaF-42KF, mol.%, H2O/O2 < 10 ppm,metallic ions < 200 ppm), suggesting the effect of W on the Cr diffusion in the alloy matrix could be ignored in the purified salts (Corrosion Science, 149 (2019) 218-225;Corrosion Science, 178 (2021) 109079). Recently, static immersion corrosion experiments of Ni-xW-6Cr alloys with W content ranging from 5 to 30 wt% were conducted in molten FLiNaK salts at 850 °C, and found the thickness of the Cr-depletion layer increases with the amount of W in the alloy, which is expected to be similar for all the Ni-xW-6Cr alloys due to the same Cr content in all the Ni-xW-6Cr alloys and the same concentration of impurities in the salts. It suggests the W addition substantially deteriorates the corrosion resistance of the Ni-xW-6Cr alloy to molten salts. According to Fick’s law, the diffusion rate of alloying element is related to its gradient of chemical potential in the alloy. And the chemical potential is a function of activity. The thermodynamic calculations show that the activities of W and Cr increase simultaneously with the increased W content, implying that the increased W content contributes to the increase in Cr and W activities, i.e., the larger chemical potentials of Cr and W in the alloy with increased W. Because metal ions in the molten salts diffuse much more quickly than that in the alloy matrix, the contents of Cr and W in the outermost alloy surface should be zero, the larger chemical potential in the alloys with higher W means higher diffusion rate of Cr and W. Furthermore, the chemical gradient of W in the corroded layer also decreases the chemical potential of Cr in the near surface, creating a larger diffusion force for Cr from the inner matrix to the surface and consequently accelerates the diffusion of Cr. It should be noted that this activity-based corrosion effect is a specific case that only can occur in high-impurities salts, which are not allowed in the molten salt reactor. Based on these conclusions, it is proposed that not only the total content of alloying elements such as Cr and Al should be controlled, but also the activity changes caused by the interaction between alloying elements should be paid attention in alloy design. The reviewer of Corrosion Science gave this work high marks, who said, “Overall this is a great study, showing how second-order effects and accounting for concentration-dependent activities can challenge our intuition about corrosion in molten salts,…the activity-based explanation by the authors is believable, and justified by the carefully conducted experiments, and it does deserve to be published in Corrosion Science following revision.”
The research was supported by the National Key Research and Development Program, the National Natural Science Foundation of China and the Youth Innovation Promotion Association, CAS.
Link: https://doi.org/10.1016/j.corsci.2021.109761