Recently, one research paper entitled “Concurrent oxygen reduction and water oxidation at high ionic strength for scalable electrosynthesis of hydrogen peroxide” was published at Nature Communications. The corresponding authors are Prof. Guntae Kim from Shanghai Institute of Applied Physics, Chinese Academy of Sciences, and Prof. Liming Dai from University of New South Wales, Australia.
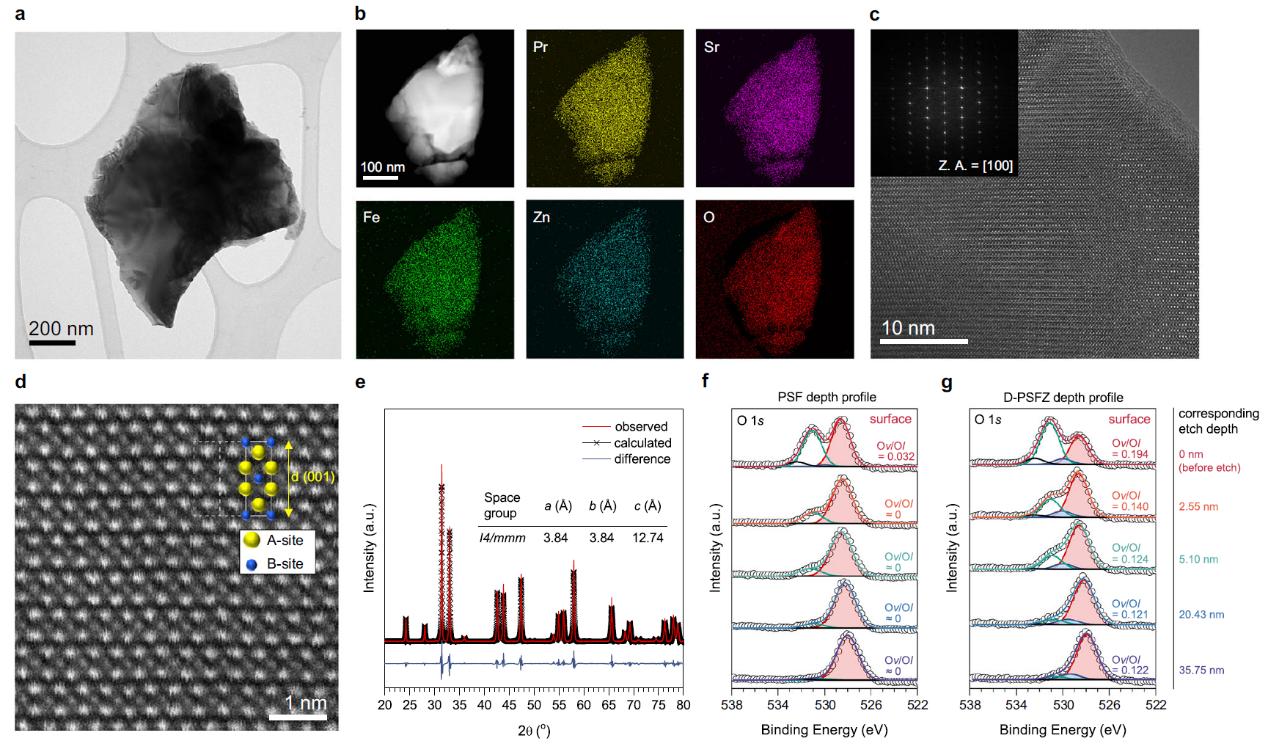
Electrosynthesis of hydrogen peroxide via selective two-electron transfer oxygen reduction or water oxidation reactions offers a cleaner, cost-effective alternative to anthraquinone processes. However, it remains a challenge to achieve high Faradaic efficiencies at elevated current densities. In this work, the researchers report that oxygen-deficient Pr1.0Sr1.0Fe0.75Zn0.25O4-δ perovskite oxides rich of oxygen vacancies can favorably bind the reaction intermediates to facilitate selective and efficient two-electron transfer pathways. These oxides exhibited superior Faradic efficiencies (~99%) for oxygen reduction over a wide potential range (0.05 to 0.45 V versus reversible hydrogen electrode) and current densities surpassing 50mA cm-2 under high ionic strengths. It further found that the oxides perform a high selectivity (~80%) for two-electron transfer water oxidation reaction at a low overpotential (0.39 V). Lastly, the researchers devised a membrane-free electrolyser employing bifunctional electrocatalysts, achieving a record-high Faradaic efficiency of 163.0% at 2.10 V and 50mAcm-2. This marks the first report of the concurrent oxygen reduction and water oxidation catalysed by efficient bifunctional perovskite oxides in a novel membrane-free electrolyser for scalable hydrogen peroxide electrosynthesis.
The research was supported by the Hundred talents program of CAS.
Link:https://doi.org/10.1038/s41467-023-41397-1.