Important Progress in Catalyst for Water Oxidation
Recently, the research team from Shanghai Institute of Applied Physics (SINAP), Chinese Academy of Sciences (CAS) has made important progress in catalyst for water oxidation. The article entitled “Novel Mechanism of Fe4+/Ni3+ Synergistic Effect via Exchange Energy Gain for Boosting Water Oxidation” has been published in the Chem Catalysis.
The development of efficient oxygen evolution reaction (OER) electrocatalysts using high activity, robust durability, and earth-abundant transition metal (TM) oxides to replace the expensive benchmark catalysts RuO2 and IrO2 is highly desirable for energy conversion and storage in modern industry.
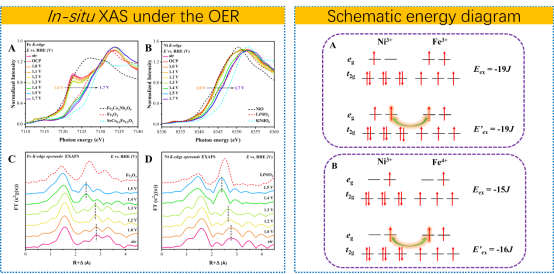
The synergistic effect of two or multi metal ions has been widely proposed to enhance OER activity in doped and multi-element catalysts, however the underlying mechanism have not been understood. In this study, Fan et al. propose a new concept for explaining the synergistic effect for OER: an increase in exchange energy of the Fe4+/Ni3+ pair, facilitated by intersite hopping.
The researchers systematically studied a series of Ni2-xFexMo3O8 (x = 0.1, 0.3, 0.5, 0.7, 1.0, and 2.0) catalysts, and surprisingly found that the best OER performance was obtained in Ni1.5Fe0.5Mo3O8 sample (reaching 196 mV at 10 mA cm-2), but not in NiFeMo3O8 with 1:1 ratio, which is expected to have an ideal synergistic effect. This implies that high OER electrocatalysis behavior cannot be attributed to a simple synergistic effect. A key aspect of this study was the observation of valence-state changes and structural reconstruction during the reaction. These changes not only enhance the electron transport between sites but also effectively improve the OER activity. The researchers further explored the mechanism behind this synergy by focusing on the exchange energy gain upon intersite hopping. They found that the transport property is significantly enhanced by this gain in exchange energy, leading to improved catalytic performance. This novel mechanism provides a deeper understanding of how Fe and Ni interact in the catalyst and how their synergy can be harnessed for efficient water oxidation. The significance of this study lies in its ability to shed light on the complex interactions between Fe and Ni in OER catalysts. This understanding can guide the rational design of more efficient catalysts for water electrolysis and other electrochemical processes.
This work was led by Prof. ZHANG Lunyong, Prof. HU Zhiwei, Prof. WANG Jianqiang and Prof. ZHANG Linjuan. Mr. FAN Yalei and ZHANG Chenjia are the first author.
Link: https://doi.org/10.1016/j.checat.2024.100981.