Recently, the research team from Shanghai Institute of Applied Physics (SINAP), Chinese Academy of Sciences (CAS) has made important progress in rational designing of catalyst for water oxidation. The article entitled “Balance between FeIV–NiIV synergy and Lattice Oxygen Contribution for Accelerating Water Oxidation” has been published in the ACS Nano.
Hydrogen obtained from electrochemical water splitting is the most promising clean energy carrier which is hindered by the sluggish kinetics of the oxygen evolution reaction (OER). Therefore, developing efficient, stable and low cost catalysts for OER are imperative for applications in renewable energy.
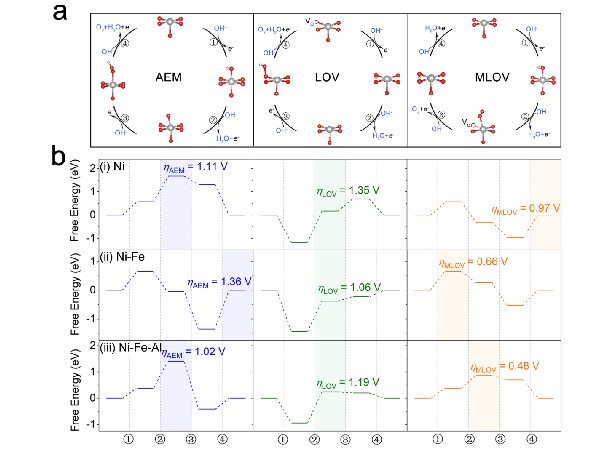
Highly active and low-cost earth-abundant 3d transition metal (TM) catalysts for industrial applications have garnered growing interest. The 3d TM catalysts with higher metal valence states have been reported to exhibit high OER activity, benefitting from the efficient intermediate adsorption and charge transfer process during the catalytic reaction. Furthermore, the high metal valence states and formed O 2p holes raise the barrier for electron removal from metal sites, facilitating the oxidation of oxygen sites. The enhanced activity of oxygen sites would promote OER pathways involving lattice oxygen. These pathways involving lattice oxygen are considered as more efficient OER routes than the conventional adsorbate evolution mechanism. However, in pathways involving lattice oxygen oxidation related to high oxidation state, the massive oxygen vacancies can be created under high overpotentials. This can lead to the destabilization of the catalyst structure, thereby a decrease in OER activity. Therefore, the strategies to enhance OER performance in 3d TM catalysts for industrial applications involve a balancing between valence states of 3d TM and lattice oxygen stability.
The researchers have found that Al doping in nano-sheet Ni-Fe hydroxide exhibits twofold advantage: (1) a strong enhanced OER activity from 277 mV to 238 mV at 10 mA cm-2 as the Ni valence state increases from Ni3.58+ to Ni3.79+ observed from in-situ X-ray absorption spectra. (2) Operational stability is strengthened, while weakness is expected since the increased NiIV content with 3d8L2 (L denotes O 2p hole) would lead to structural instability. This contradiction is attributed to a reduced lattice oxygen contribution to OER upon Al doping, as verified through in-situ Raman spectroscopy, while the enhanced OER activity is interpreted as an enormous gain in exchange energy of FeIV-NiIV, facilitated by their intersite hopping. This study reveals a mechanism of Fe-Ni synergy effect to enhance OER activity, and simultaneously to strengthen operational stability by suppressing the contribution of lattice oxygen.
This work was led by Prof. LI Da-Wei, Prof. HU Zhiwei and Prof. ZHANG Linjuan. Dr. JING Chao and Dr. LI Lili are the first author.
Link: https://pubs.acs.org/doi/10.1021/acsnano.4c01718