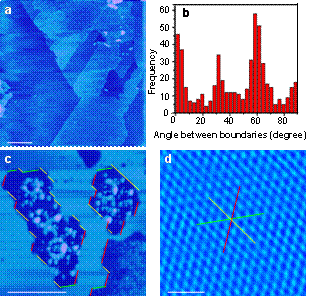
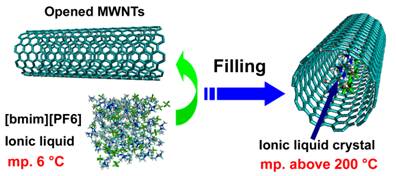
Room temperature ionic liquids (RTIL) have received much attention due to their importance for a broad range of applications. However, little is understood about the microstructure and phase behavior of RTIL on solid surface. Using atomically flat mica as substrate to adsorb [bmim][PF6], or (1-butyl-3-methylimidazolium hexafluorophosphate), a prototype ionic liquid, we directly observed by AFM the coexistence of solid and liquid phases of the [bmim][PF6] on the mica surface at room temperatures[1]. In addition, in studying the phase behavior of [bmim][PF6] inside multi-walled carbon nanotube (MWNTs), we found that [bmim][PF6] underwent a fully different phase transition and crystal formation when confined in MWNTs, leading to the formation of a stable polymorphous crystal with a melting point of over 200 °C[2]. The findings are of help in understanding phase transitions of materials confined in nanoscale environments. The work was highlighted by C&EN in Science and Technology Concentrate (Feb. 19, 2007).
|
|
References
[1] Yaodong Liu, Yi Zhang, Guozhong Wu, Jun Hu. J. Am. Chem. Soc., 2006, 128, 7456-7457.
[2] Shimou Chen, Guozhong Wu, Maolin Sha, Shirong Huang. J. Am. Chem. Soc., 2007, 127, 2416-2417. |