






Unravelling the Role of the Compressed Gas on Melting Point of Confined Ionic Liquid
Recently, scientists from Prof. Guozhong Wu’s group at Shanghai Institute of Applied Physics, Chinese Academy of Sciences, have successfully demonstrated that the confined ionic liquid under high-vacuum condition formed a crystalline-like phase. The result was published by JPCL. (Unravelling the role of the compressed gas on melting point of liquid confined in nanospace, J Phys Chem Lett, 2012, 3: 1052?1055.)
Room-temperature ionic liquids (ILs) are a special class of liquids solely composed of cations and anions. ILs are usually liquid at room temperature but have an extremely low vapor pressure. Therefore, they provide a good model liquid for handling in high vacuum. This is very important for the preparation of the genuine confined liquid by filling them into a nanospace; as the filling process can be done under vacuum condition, the final obtained confined liquids in composites are free from compressed gases and other volatiles.
In this study, we have sought a simple means by which to encapsulate ILs so that they are completely confined in the cavities of porous nanoparticles. Complete nanoconfinement of ILs was realized by heating a mixture of IL and mesoporous silica oxide particles (hereafter referred to as SiO2) at high temperature under high vacuum. We found that the complete filling and close packing of the IL act as the dominant factors in the elevation of the melting point of the confined ILs. In contrast, when the IL is immobilized onto the surface of SiO2 by filling at atmospheric pressure, the melting point of the IL is decreased in comparison with that of the bulk (Scheme 1).
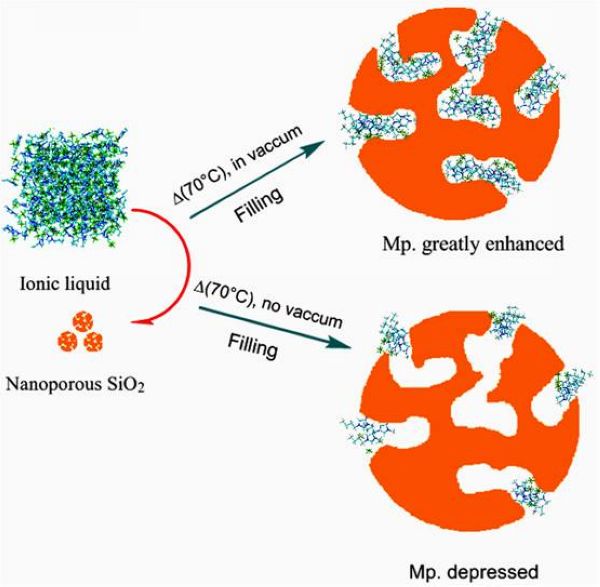
Scheme 1. Schematic Diagram of the Change in Melting Point of an IL Entrapped by Mesoporous Silica Oxide Particles under Vacuum Conditions versus that at Atmospheric Pressure.
The phase behavior of the ILs obtained by the two different filling procudures was investigated by differential scanning calorimetry (DSC) and thermal gravity (TG) analysis. The result showed that filling under vacuum leads to a significant increase in the melting point. Transmission electron microscopy (TEM) was used to characterize the confined IL in mesoporous SiO2. However, the conventional TEM mode can not give the difference of mesoporous silica oxide particles before and after confinement. Fortunately, the IL in the nanocomposites can be discerned in the atomicresolution high-angle annular dark-field (HAADF) images by scanning electron probe STEM mode due to the existence of heavy-atom iodine in [AMIM][I]. As shown in Figure 2, the confined IL in the [AMIM][I]@SiO2 sample reveals clearly that some IL is encapsulated in the channel of SiO2 (indicated by the white spots in Figure 2c). The energy dispersive X-ray (EDX) spectra confirmed the white spots are only present in the [AMIM][I]@SiO2 sample, indicating that the molecular packings of the [AMIM][I] in the two samples are much different. The confined IL obtained by conducting the filling process under high-vacuum condition (ie, [AMIM][I]@SiO2 sample) shows strong signals of the heavy atom of iodine, which should be due to the formation of a crystalline-like phase of the IL in the completely confined space. In brief, by the use of the nonvolatile property of ILs, we first realized encapsulation of ILs inside of mesopores of SiO2 nanoparticles with a complete removal of the compressed gas by loading at high vacuum. It was found that the compressed gas plays an important role in the packing and phase behavior of the confined ILs. Without compressed gas, the nanoconfinement truly led to a considerable increase in the melting point of the confined ILs.

Figure 2. HAADF-STEM images and the corresponding EDX spectra of mesoporous SiO2 (a, d), [AMIM][I]/SiO2 (b, e), and [AMIM][I]@SiO2(c, f). The scale bare in (c) applies to all three images.