[CTi72+]: Seven Coordinate Carbon
Recently, Prof. Yi Gao at Shanghai Institute of Applied Physics, Chinese Academy of Sciences has demonstrated the stability of heptacoordinated carbon system [CTi72+] using computer simulations with the collaborators. This work has been published by The Journal of Physical Chemistry Letters ([CTi72+]: Heptacoordinate Carbon motif? J. Phys. Chem. Lett. 2012, 3, 2264-2268). On August 14, “Chemistry World” from Royal Society of Chemistry presented a cover story entitled with “Carbon clusters score lucky seven” to report this latest advancement.
It is well-known that carbon atom has 4 valence electrons, thus it could form 4 covalent bonds. However, the isolation and characterization of CH5+ in 1952 arouses the great interest of hypercoordinate carbon in the last half century. The discovery of carbon with maximum coordination becomes one of the most challenging subjects in physical organic chemistry. Until now, the maximum number is six for experiments. On the other hand, the hypercoordinate carbon systems with six or more coordination proposed by theoretical chemists are not stable because they have lower-energy isomers.
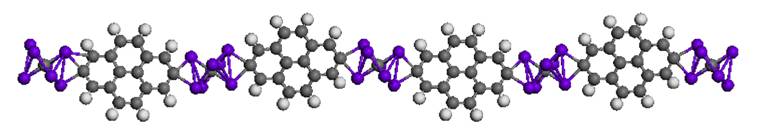
In this paper, Prof. Gao and collaborators proposed a highly stable seven-coordinated carbon dication [CTi72+]. This system is formed with a planar pentacoordinate carbon with five titanium atoms in-plane and two titanium atoms out-plane (Figure 1). Based on the basin-hopping global-minimum search, this system is lower in energy than its isomers. And it has high symmetry (D5h), large HOMO-LUMO gap (>1 eV) and low charge. This is the maximum coordination number for stable hypercoordinate carbon systems ever discovered until now. Further simulations confirmed this dication could exist stably at the room temperature which would likely be synthesized in the lab. They also proposed a quasi-one dimensional nanowire based on this motif (Figure 2). Prof. Henry Rzepa from Imperial College London comments: “New ideas are as likely to come from computation as from experiment, and there is a vibrant, fruitful, interplay between these, The CTi72+ system here is a wonderful example of how this process happens. The strength of ideas such is that it inspires experimental chemists to go make it in the lab.”
This research is carried out by the scientists from Shanghai Institute of Applied Physics, University of Nebraska-Lincoln, Brookhaven National Lab, Hefei University of Technology and Harbin University of Science and Technology. This work is supported by Shanghai Institute of Applied Physics, Supercomputing Center of Chinese Academy of Sciences in Beijing and National Supercomputing Center in Shenzhen.Figure 1
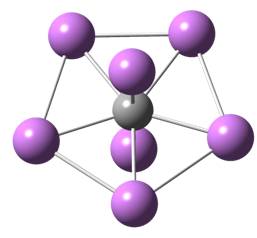
Figure 2