






Is polar surface always hydrophilic?
The wetting properties of surfaces, named hydrophobicity and hydrophilicity, play a critical role in extensive physical, chemical, biological and/or engineering sciences, such as micro-fluids devices design, biosensors and fabricating superhydrophobic self-clearing materials, protein folding and self-assemble of amphiphiles. The misleading of the surface hydrophobicity and hydrophilicity would lead to the misunderstanding of the interactions and dynamics property at the interface, thus greatly affect the analysis and the design of the experiments.

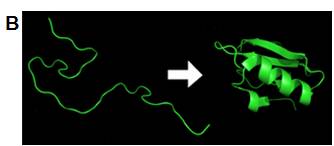
Fig 1 A. “Lotus effect” with a water droplet on the superhydrophobic surface and B. Protein folding process driven by the hydrophobic effect
As a polar molecule, water molecule usually has a high affinity with other polar molecules/groups or ions. Namely, the surfaces with polar molecules or groups are usually easily hydrated by water, regarded as hydrophilic. Actually, modifications of polar groups are always chosen by the experimental scientists to create the hydrophilic materials.
However, is the polar surface always hydrophilic? Recently, Dr. Chunlei Wang and Prof. Haiping Fang at Shanghai Institute of Applied Physics, Chinese Academy of Sciences, have shown that the polar surface may still be hydrophobic. They found that the length of the charge dipoles (the distance between a positive charge and its nearest negative charge) on the solid surface played a key role on the wetting behavior. Both the theoretical analysis and the molecular dynamics simulations show that there is a critical length of the charge dipoles on the solid surface, below which the charge dipoles on the solid surface plays unexpectedly negligible role in the wetting property. The solid surface still exhibits hydrophobic behavior when the dipole length is less than the critical value, indicating that the water molecules on the solid surface seem not to “feel” attractive interactions from the charge dipoles on the solid surface, no matter how large the moment magnitude of the charge dipoles is.When the charge dipole lengths are greater than this critical length, it they is found that the surfaces become more hydrophilic with the increasing of the dipole lengths increase. Those results have been published on Scientific Reports (Nature) (Sci. Rep., 2012, 2, 358) (http://www.nature.com/srep/ 2012/120411/srep00358/full/srep00358.html).
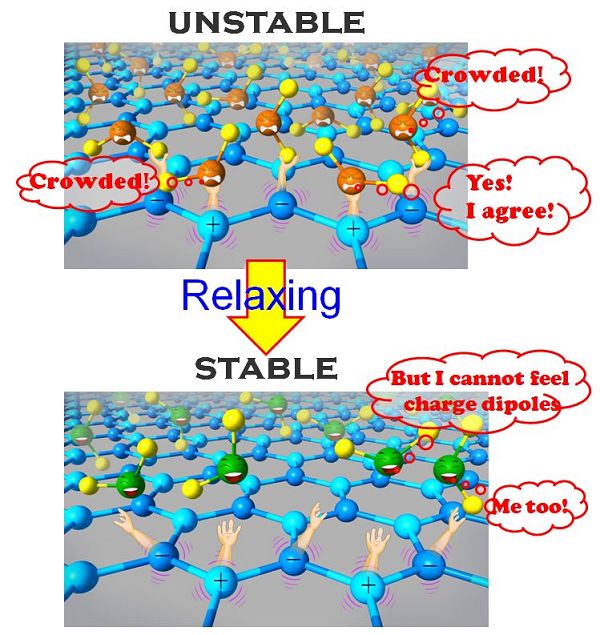
Fig 2. Upper Figure: Oxygen atoms of water (orange crying face) and the hydrogen atoms of water (yellow balls) are bound by the positive and negative charges on the surface. When the distance between surface charges is small, the water molecules would be very crowded and the state is unstable.
Bottom Figure: When the water molecules relax themselves and distance between water molecules becomes large, the system becomes stable. However, it becomes impossible that those hydrogen atoms (yellow balls) stay very close to the negative surface charge while those oxygen atoms (green smile faces) stay very close to the positive surface charge. This leads to the result that water molecules may not be able to feel the attractions from the charge dipoles, making the surface hydrophobic.
Those unexpected observations result from the collective interactions between the water molecules and charge dipoles on the solid surface, where the steric exclusion effect (crowded effect) between water molecules prevents those hydrogen atoms of water molecules from staying very close to the negative charge and those oxygen atoms of water molecules from staying very close to the positive charge, reducing the interactions between the water molecules and the charge dipoles. Interestingly, the steric exclusion effect was also important for surfaces with charge dipole lengths greater than this critical length. When the charge dipole lengths are greater than this critical length, it is found that the surfaces become more hydrophilic with the dipole lengths increase. Further analysis shows that existence of the critical length is universal for various types of surface. We note that , very recently, C. L. Wong in the University of California, Los Angeles, have experimentally (Phys. Rev. E 2012, 85, 031501) shown that the dipoles with extremely high dipole density on the surface plays negligible role on the surface water wetting behavior on the basis of inelastic x-ray scattering techniques, consistent with our theoretical prediction.
At the year 2009, this research group have proposed that a stable liquid water droplet on a monolayer may emerge on a well-defined charged solid surface at room temperature, which is termed of “water that does not completely wet water” (Phys. Rev. Lett., 2009, 103, 137801; J. Phys. Chem. C, 2011, 115, 3018).This prediction has also been confirmed by the experimental results of the German research group at 2010 (Adv. Colloid Interface Sci, 2010, 157, 61) and the Australia research group at 2011 (Soft Matter, 2011, 7, 5309;Langmuir, 2011, 27, 10753). Very recently, the behavior of “water that does not completely wet water” have also been observed by MD simulations on Talc surfaces(J. Am. Chem. Soc. 2011, 133, 20521) and hydroxylated alumina and hydroxylated silica surfaces(J. Phys. Chem. C 2012, 116, 15962), suggesting the unrecognized generality of this phenomenon in this world in our prediction. All of these works draw a relatively complete picture onhow the surface charges and charge dipoles affect the wetting property and have clearly shown that the polar surfaces may be unexpected hydrophobic, contrary to our common sense.
This work was performed in cooperation by researchers at Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai University, Sichuan University and Zhejiang University in China. The work is partially supported by Shanghai Leading Academic Discipline Project, the Knowledge Innovation Program of the Chinese Academy of Sciences, the National Natural Foundation of China and Shanghai Supercomputer Center of China.