Interplay between Water and oxygen vacancy on TiO2 anatase
TiO2 is an important semiconductor with application in a wide range of areas, such as degradation of toxic organic pollutants, water splitting, and solar cells. Its interaction with water has brought a particular research interest because most of reactions take place at aqueous surroundings. Graduate student Yadong Li under supervision of Prof. Yi Gao from Shanghai Institute of Applied Physics, Chinese Academy of Sciences, recently discovered a novel interplay between water and subsurface oxygen vacancy (Vo) on TiO2 anatase (101) surface. Their relevant research has been recently published on Physical Review Letters (Phys. Rev. Lett. 112, 206101 (2014)).
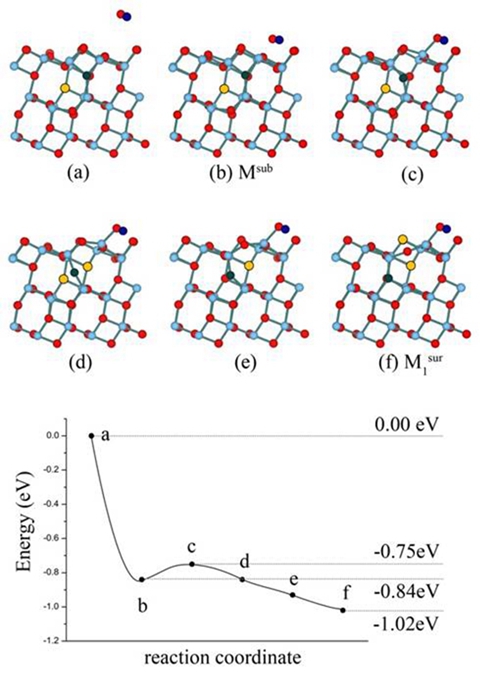
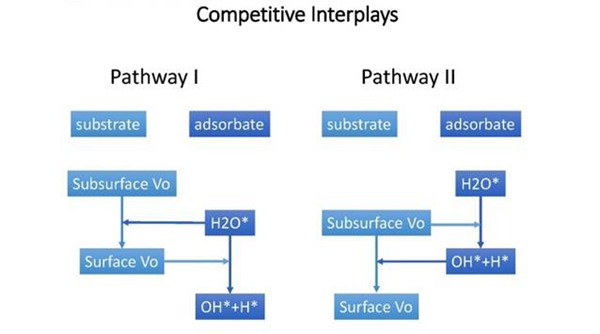
It has long been considered the high activity of TiO2 surface is attributed to the surface Vos. In recent years, scientists found that the subsurface Vos are more stable than surface Vos, and the surface Vos will diffuse to the bulk at temperature as low as 200K. This makes it hard to understand the high activity of anatase.By utilizing density-functional calculation, Li and Gao found that the relative stability of surface and subsurface Vo will reverse after water adsorption, and the adsorbed water can induce subsurface Vo to bubble up to the surface layer, which further facilitate water dissociation. In another energetically competitive pathway, molecular water dissociates over subsurface Vo first, followed by barrierless Vo diffusion from subsurface to surface. This mechanism indicates that not only surface Vos, but subsurface or even bulk Vos can contribute to the activity of anatase. This novel interplay between adsorbate and substrateprovide a new perspective way to understand the high catalytic activity of anatase (101) surface, and may help us understand the high catalytic activity of other metal oxides.
This work is supported by Chinese Academy of Sciences, Shanghai Institute of Applied Physics, National Natural Science Foundation of China and Science and Technology Commission of Shanghai Municipality. The computational resources utilized in this research were provided by Shanghai Supercomputer Center, National Supercomputing Center in Tianjin and Supercomputing Center of Chinese Academy of Sciences in Beijing.