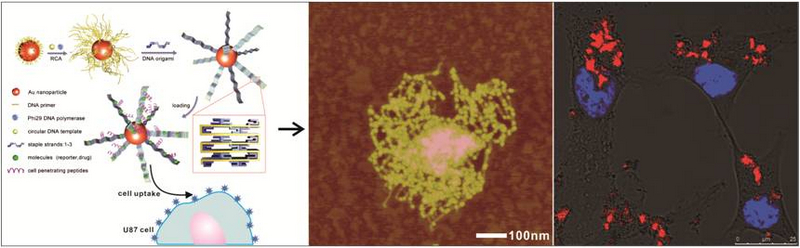
Inorganic nanoparticles have attracted considerable attentionas nanocarriers for cellular imaging and drug deliveryowing to their unique properties, such as their high surface-to-volumeratio, optical behavior, and the ability to be functionalizedwith biomolecules. However, many studies haveshown that the practical use of nanoparticles as nanocarriersis often hampered either by the low molecule-loading
capacity of small nanoparticles owing to their limited surfaceareas or by the low-efficiency cellular uptake and lowintracellular stability of large nanoparticles.Chunhai Fan and Shiping Song at Shanghai Institute of Applied Physics, Chinese Academy of Sciences and co-workers have now developed a novel three-dimensional (3D) superstructure based on the growth and origami folding of DNA on gold nanoparticles (AuNPs). The 3D superstructure contains a nanoparticle core and dozens of two-dimensional (2D) DNA belts folded from long single-stranded DNAs grown in situ on the nanoparticle via rolling circle amplification (RCA). The authors designed two mechanisms to achieve the loading of molecules onto the 3D superstructures. In one mechanism, ligands bound to target molecules are merged into the growing DNA during the RCA process (merging mechanism). In the another mechanism, target molecules are intercalated into the double-stranded DNAs produced by origami folding (intercalating mechanism). They demonstrated that the as-fabricated 3D superstructures have a high molecule-loading capacity and that they enable the high-efficiency transport of signal reporters and drugs for cellular imaging and drug delivery, respectively. The work was published recently on journal of Angew. Chem. Int. Ed. (2015, 54, 2431 –2435).