Janus effect of antifreeze proteins on ice nucleation
Antifreeze proteins (AFPs) protect a broad range of biological organisms inhabiting subzero environments via controlling ice formation. Since the discovery of AFPS in 1960s, a vast body of experimental and theoreticalworkshavebeen undertaken to investigate the molecular level mechanism of the AFPs in controlling ice formation. However, the exact effect of AFPs on ice nucleation is under intense debates.
Based on the fact that ice-binding face(IBF) and non-ice-binding face (NIBF)of the AFPS possess distinct functional groups, Prof. Jianjun Wang fromInstitute of Chemistry, Chinese Academy of Sciences(CAS), Dr. Chunlei Wang and Prof. Haiping FangfromShanghai Institute of Applied Physics, CAS, and Prof. Ji Ma fromXinjiang Universityinvestigated the effect of IBF and NIBF of AFPs on ice nucleationbyselectively tethering IBF and NIBF of AFPs to solid substrates.Theexperimentalresults revealed that the IBF of AFPs facilitates ice nucleation, whereas the NIBF depresses ice nucleation (shown in Fig. 1).
The results of molecular dynamics simulations showedthat there are distinct molecular hydrationwater structures around IBF and NIBF (Fig. 2). For the IBF of AFPs, water molecules form ice-like interfacialwater structure due to the special arrangement of hydrophobic methyl and hydrophilic hydroxyl groups on the IBF.In strong contrast, almost no ice-like water structure is formed on the NIBF, which is possibly due to the absence of regular hydrophobic/ hydrophilic patterns as well as the existence of charged groups and bulky hydrophobic groups. This helpsestablish the molecular level understanding of AFPs in tuning ice nucleation.
This work provides a comprehensive picture of the effect of AFPs on ice nucleation, which will certainly guide materials scientists to design and synthesize biomimetic compounds for regulating ice nucleation.The work has been published on PNAS, 2016, doi: 10.1073/pnas.1614379114.
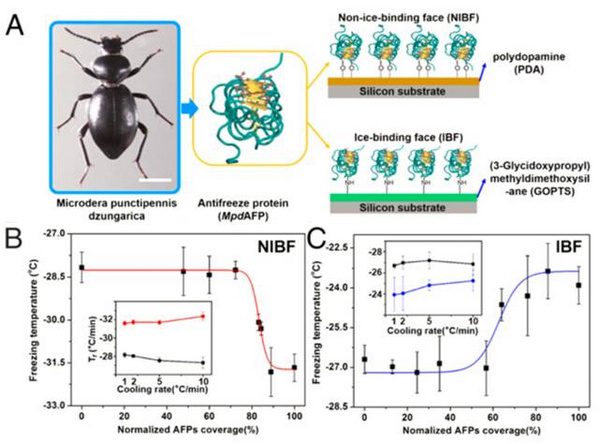
Fig 1:Experimental methods through the tethered AFPS on PDA and GOPTS surfaces, revealthat IBF of AFPs facilitates ice nucleation, whereas the NIBF depresses ice nucleation, named “Janus effect”.
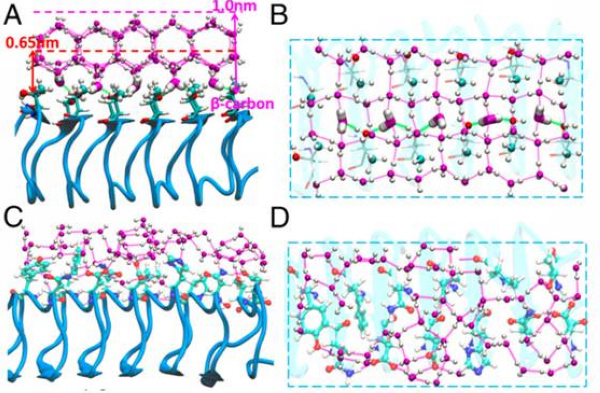
Fig 2. Molecular dynamics simulations reveal that there are distinct molecular hydration water structures around IBF and NIBF, providing the possible molecular level understanding of AFPs in tuning ice nucleation.

