






Recently, the alloy research team of Shanghai Institute of Applied Physics (SINAP), Chinese Academy of Sciences (CAS) made progress in the high-temperature corrosion mechanism of Ni-28W-6Cr in molten salts and proposed a mechanism that Mo ion impurities-induced corrosion of W-containing Ni-base alloy. The mechanism answers the question that W was unexpectedly corroded by the molten salt that should be immune to molten salt, which has been troubling the alloy researchers for molten salt reactor since the 1960s. The research result entitled “Metallic impurities induced corrosion of a Ni-26W-6Cr alloy in molten fluoride salts at 850 oC” was published in Corrosion Science. The first author is Dr. Hua Ai, and the corresponding author is Dr. Xiang-Xi Ye.
Ni-28W-6Cr alloys, developed by SINAP, have been considered as one of the potential advanced structural materials for molten fluoride salt techniques due to their excellent high temperature mechanical performance. However, the influence of W addition on the corrosion behavior of Ni-based alloys is still controversial. In theory, W is difficult to be fluorinated and the diffusion rate of W in nickel matrix is much slower than other common metal elements, which are beneficial to the corrosion resistance of the Ni-based alloys to molten fluoride salts. Nevertheless, some previous studies by the researchers from Oak Ridge National Laboratory (ORNL) and Centre national de la recherche scientifique (CNRS) showed that Ni-based alloys containing W suffered unexpected dissolution of W in the molten fluoride salts, which could not be explained. Our previous study showed that Ni-26W-6Cr alloy presents high compatibility with purified FLiNaK salts at 800 oC and little depletion of W from the alloy (Corrosion Science 149 (2019) 218-225). Therefore, we suspected that the "unexpected" dissolution of W is caused by the impurities in the molten salt. Besides, the researchers from CNRS indicated that Fe2+ ions in the salts cause the corrosion of W. To verify the effect of impurities on the corrosion of W, FLiNaK salts with Fe and Mo impurities were applied to evaluate the corrosion of Ni-28W-6Cr alloy at 850 ℃. The results show that Fe2+ impurities cannot corrode W in the alloy, but Mo3+ can react with W by the reaction W+1.333MoF3=WF4+1.333Mo. It can explain the ORNL results of the unexpected dissolution of W from Ni-Mo alloy in the LiF-NaF-KF-UF4 salts. It was caused by the dissolution of Mo from the alloy bulk, which is induced by impurities such as water, an then Mo impurity further reacts with W on the alloy surface. Given the significant effect of W on the neutron spectra of reactors, the Mo impurity in the fluoride salts should be strictly limited for the application of the alloys containing W, including the Ni-26W-6Cr alloy.
The research was supported by the National Natural Science Foundation of China and the Youth Innovation Promotion Association, CAS.
Link: https://www.sciencedirect.com/science/article/pii/S0010938X2032360X?via%3Dihub
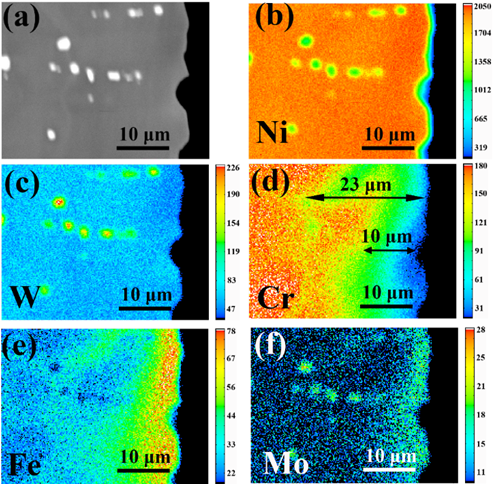
Fig.1 Chemistry of cross section specimen after molten salt corrosion at 850 oC.