






Progress in improving the high-temperature oxidation resistance of Ni-28W-6Cr for molten salt reactor by doping to hinder the oxygen vacancy-mediated oxidation
Recently, the alloy research team of Shanghai Institute of Applied Physics (SINAP), Chinese Academy of Sciences (CAS)made important progress in the research on the high-temperature oxidation resistance of Ni-28W-6Cr alloy, and proposed an approach to improve the high-temperature oxidation resistance of Ni-28W-6Cr alloy by doping to hinder the oxygen vacancy-mediated oxidation. The research result entitled “An approach to improve the oxidation resistance of a Ni-28W-6Cr alloy by hindering the oxygen vacancy-mediated oxidation” was published in Corrosion Science. The first author is PhD student Shulin Liu, and Prof. Xiang-Xi Ye, Prof. Xingtai Zhou, and Dr. Chen Ming (from Shanghai Institute of Ceramics, CAS) are the co-corresponding authors.
The reliability of structural materials in aggressive environments is a paramount safety and economic concern for developing new-generation energy systems. For molten salt reactors, the structural alloys are exposed to both high-temperature air and molten salts. Because oxide-forming elements (Cr, Al, Si, etc.) are easy to be attacked by molten salts, and refractory elements (Ni, W, Mo) are immune to molten salts, Ni-based superalloys developed for molten salts powered energy system are rich in Mo, W and have low content of Cr to keep proper oxidation resistance. In the pursuit of energy efficiency, the temperature of these energy systems continues to increase. The proportion of solid solute strengthening refractory metals, such as W and Mo, in the superalloys constantly rises to improve their mechanical performance at elevated temperatures, which is harmful to their high-temperature oxidation resistance. For example, Ni-28W-6Cr alloys, developed by SINAP, have been considered one of the potential advanced structural materials for molten fluoride salt techniques due to their excellent high temperature mechanical performance corrosion resistance to molten salts. However, the previous work showed that the oxidation resistance of Ni-xW-6Cr alloy deteriorates dramatically when the content of W reaches above 25 wt.%, because the excessive W can prevent the formation of compact NiCr2O4 oxide scales. The loose inner oxide scale of NiWO4 with a large number of oxygen vacancies provides paths for the inward diffusion of oxygen (Corros. Sci., 149 (2019) 87-99). To improve the high-temperature oxidation resistance of Ni-28W-6Cr alloy, hindering the formation of oxygen vacancies in the inner oxide scales may be an effective approach.
NiWO4 is a common oxide scale in the W-bearing Ni-based superalloys. The valence states of Ni, W and O in NiWO4 are +2, +6 and -2, respectively, and the oxygen vacancies are +2-charged. If a +2-charged nickel atom in NiWO4 is replaced a dopant with a higher valence, such as +4 dopant, the dopant will carry an extra +2 charge compared to Ni, and is expected to inhibit the formation of +2-charged oxygen vacancies or repel them, thus hindering their diffusion. With the above idea, i.e., doping a high valence metal element into the oxides to hinder the formation of oxygen vacancies, the alloy research team recently added Zr into the Ni-28W-6Cr alloy to improve its oxidation resistance. The results showed that the oxidation resistance of the Ni-28W-6Cr alloys was significantly enhanced with the Zr addition.The thickness of the inner oxide scales in the Ni-28W-6Cr (the main component is NiWO4) significantly decreases accordingly. The oxide scales were analyzed by using a combination of synchrotron radiation-based micro X-ray fluorescence (μ-XRF), micro XRD (μ-XRD), and micro X-ray absorption near edge structure (μ-XANES). No Zr oxide (ZrO2) was detected in all the oxide scales of Zr-bearing Ni-28W-6Cr alloy, and Zr charged +4 prefers to segregate in the inner NiWO4. DFT calculations showed that Zr tends to replace Ni in the NiWO4 , rather than W or locate at the the interstitial site. Then, the defect formation energy of ZrNi (Zr in Ni sites) and oxygen vacancy VO with a variation of Fermi energy was calculated. In the p-type or neutral region, both ZrNi and VO tend to be ionized and ZrNi owns lower formation energy than VO. As ZrNi donates electrons to the system, the Fermi energy tends to shift to the n-type region, which increases the formation energy of VO. Therefore, the Zr dopants in the NiWO4 could significantly reduce the concentration of oxygen vacancy, which leads to the reduction of oxygen transport channels and thus enhances the oxygen resistance of the alloys.
The research was supported by the National Key Research and Development Program, the National Natural Science Foundation of China and the Youth Innovation Promotion Association, CAS.
Link: https://doi.org/10.1016/j.corsci.2021.109480

Fig. 1 μ-XRF mapping of Ni (a) and Zr (b) in the Ni-28W-6Cr-0.2Zr alloy cross section; (c) - (g) are the μ-XRD patterns; μ-XANES spectra (×1000) of Zr in the oxide scale and alloy matrix of Ni-28W-6Cr-0.2Zr.
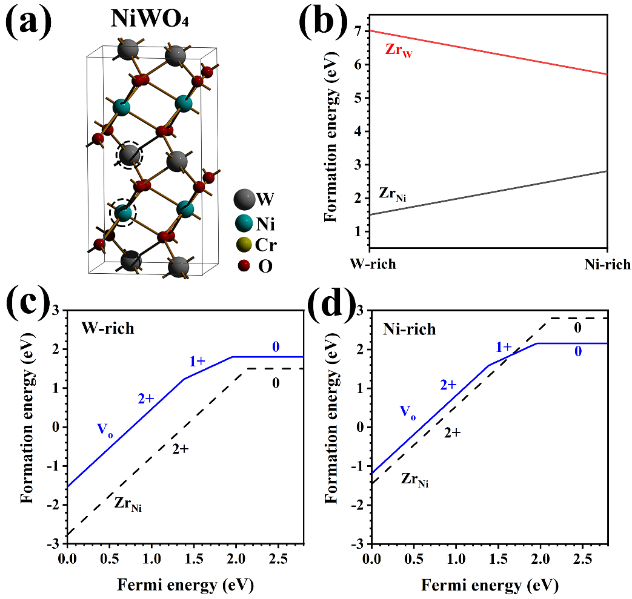
Fig. 2 (a) Crystal Structure of NiWO4; (b) The defect formation energy of ZrNi (Ni site) and ZrW (W site) as a function of chemical potential. (c) and (d) denote the defect formation energy of ZrNi and VO (oxygen vacancy) as a function of Fermi energy under W- and Ni-rich conditions, respectively.