






1. We independently set up a high-temperature in-situ UV-Vis absorption Spectrometer (HT UV-Vis Spectrometer, Fig. 1), which can measure the absorption spratra of molten salts within the wavelength range of 200-950 nm at high temperature (800 ℃). It can be used not only for the detection of metal ions in molten salts but also for the determination of the absorbance of focused solar fused salt. Three invention patents based on this technology have been granted. According to the principle of laser heating technique, HT NMR, which is suitable for the in-situ detection of molten salt system, has been developed and can detect the NMR signals of various elements at 900 ℃, thus laying a foundation for the characterization of the structure and reaction dynamics of in-situ molten salt at high temperature.

Fig. 1 High temperature UV-Vis device
2. The high-temperature in-situ UV-Vis device could be used to conduct the in-situ detection of the corrosion reaction of chromium and Cr3+ ions in molten fluoride salt, and the corrosion mechanism of chromium in molten salt was described from the microstructure level. The experimental evidence for the view that the corrosion of metal ions in fluorine acid molten salt was weaker. (Fig. 2) The results showed that, compared with molten FLiBe salt, the free F- ions in molten FLiNaK could promote the decomposition of Cr2+ ions at the interface of molten salt and regenerate highly corrosive Cr3+ ions, thus enhancing the corrosion of metal Cr in molten salt.
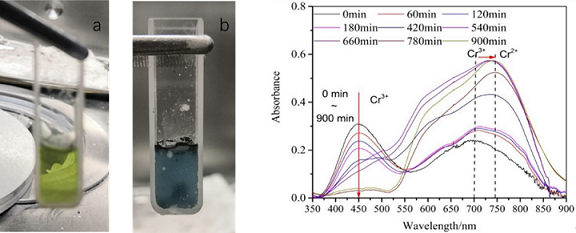
Fig. 2 Pictures of Cr in FLiBe-CrF3 fused salt before and after the reaction and the UV absorption spectrum curve with time
3. The in-situ structure of FLiNaK salt at high temperature was studied by using the HT-NMR technology, and the microstructural transformation of FLiNaK salt from liquid melting at high temperature to solid state at room temperature were described in detail. It was found that Na+ ions would be doped in KF lattice during the cooling process, which broke the previous cognition that continuous solid solution couldn’t be formed due to the significant difference of the lattice constants of LiF/NaF/KF.
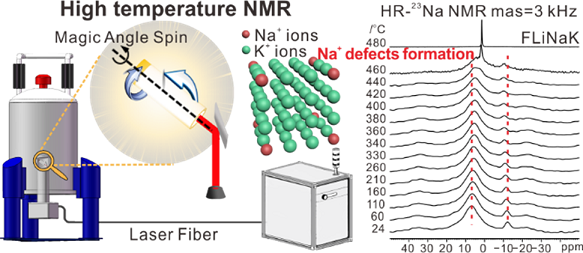
Fig. 3 Principle of high temperature NMR spectrometer and 23Na NMR spectra of Na-K ion doping structure at variable temperatures
4. The reaction process of metal beryllium with different molten fluoride salts (FLiNaK/FLiBe) were studied, and it was found that metal beryllium reacted violently with molten FLiNaK. The metal Be with low reducibility would have reaction in molten salt and generate alkali metal with high reducibility, but metal Be wouldn’t have an obvious reaction with molten FLiBe and LiCl-KCl salts. Combining with high-resolution NMR and infrared spectroscopy, the reaction mechanism of metal Be and FLiNaK was described, and the results showed that the free F- ions in molten salt reacted with metal Be at the interface to generate intermediates and ultimately promote the overall reaction.
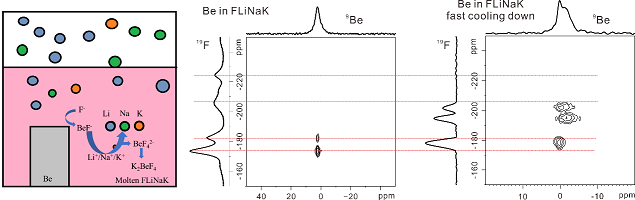
Fig. 4 Diagram of the reaction process between beryllium metal and FLiNaK fused salt and spectrogram of reaction product 2D 19F-9Be Hetcor NMR spectra
Relevant achievements have been published in a famous journal with the title of Corrosion of Cr in fused salts with different fluoroacidity in the presence of CrF3, High-Temperature Magic-Angle Spin Nuclear Magnetic Resonance Reveals Sodium Ion-Doped Crystal-Phase Formation in FLiNaK Eutectic Salt Solidification. (Corrosion Science:2020, 169, 108636,.J. Phys. Chem. C: 2021, 125, 8, 4704–4709;) This series of research is supported by the “National Natural Science Foundation of China”, “Strategic Priority Research Program of the Chinese Academy of Sciences”, and “Young Potential Program of Shanghai Institute of Applied Physics, Chinese Academy of Sciences”.
(Provided by the Department of Molten Salt Chemistry and Engineering)
Article link: https://doi.org/10.1016/j.corsci.2020.108636 ; https://doi.org/10.1021/acs.jpcc.0c10608