






Electrochemical water splitting is a promising approach for large-scale energy storage. Because of the sluggish four-electron kinetics of oxygen evolution reaction (OER) in water splitting, it is urgent to develop highly efficient electrochemical catalysts for OER. The concept of synergistic effects is extensively employed in the design of electrocatalysts and explaining the electrochemical reaction. However, synergistic effects have not been properly described and are often misinterpreted. There are two main reasons. First, the geometric and electronic structure of the catalyst may change under OER reaction conditions, so it is necessary to identify the real active sites of catalysts. In addition, most materials achieve synergistic effects mainly through interface engineering, such as for intermetallic/interface, structurally ordered multi-metallic compounds, core-shell nanoparticles, heterostructures, etc., but their synergistic effect of the active sites occur restricted only on the grain boundaries or phase interfaces. Thus, developing catalysis with maximizing synergistic effects and demonstrating the mechanism of synergistic effects, will provide a perspective on designing high-efficiency catalysis.
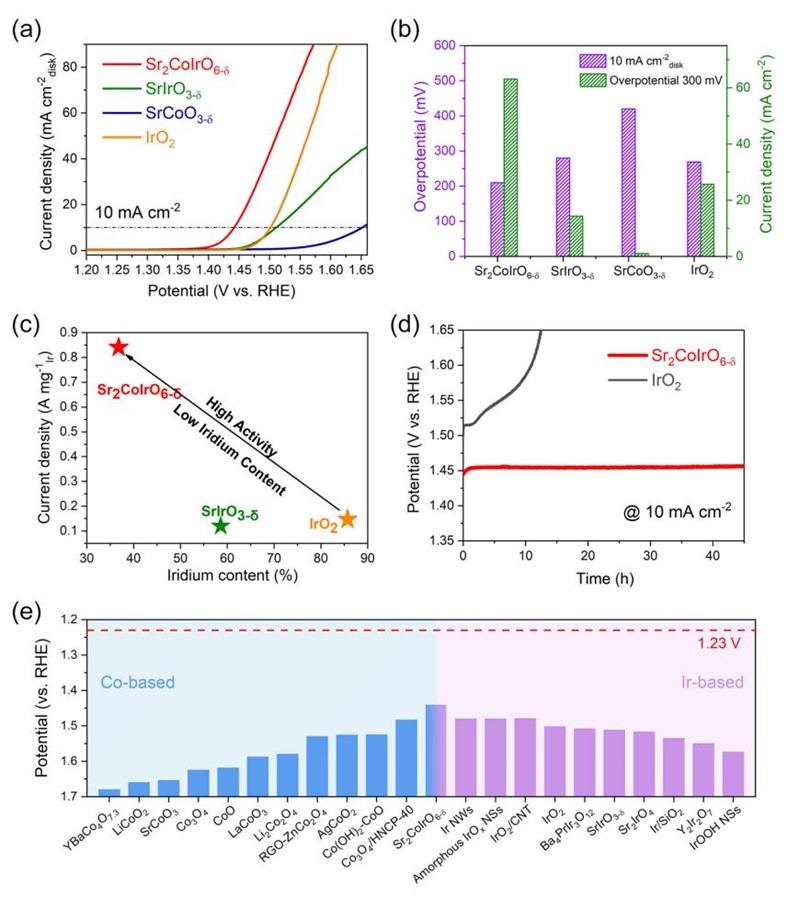
Figure 1. Electrocatalytic properties of the studied electrocatalysts for OER.
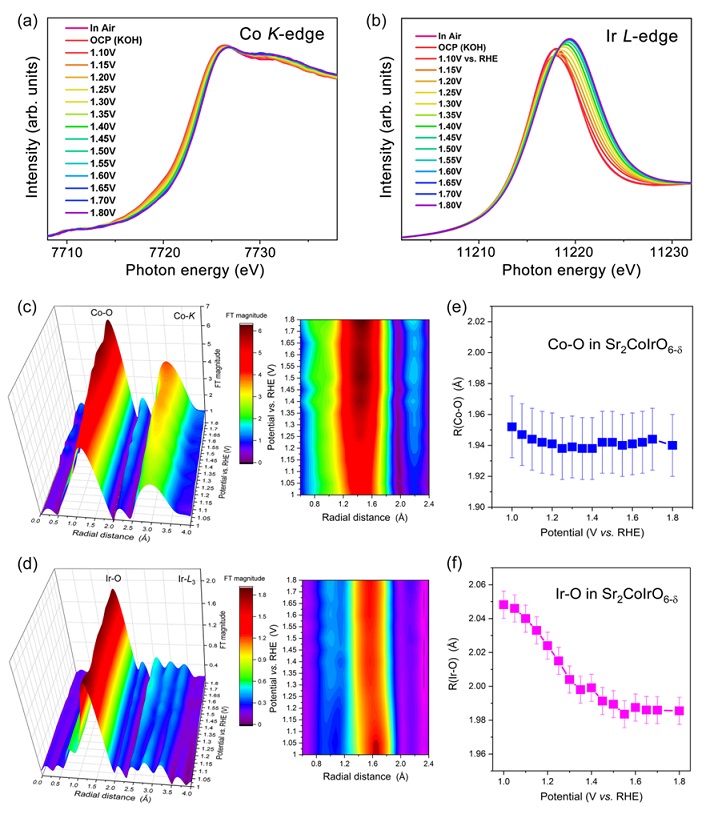
Figure 2. Fast operando spectroscopic characterizations.
The co-first authors are post-doctorate Lili Li and Hainan Sun, and the co-corresponding authors are Prof. Jianqiang Wang, Prof. Linjuan Zhang, and Prof. Zhiwei Hu (from the Max Planck Institute for Chemical Physics of Solids).
The research was supported by “Transformational Technologies for Clean Energy and Demonstration,” Strategic Priority Research Program of the Chinese Academy of Sciences, the K. C. Wong Education Foundation, the National Science Foundation of China, Youth Innovation Promotion Association, Chinese Academy of Science, Instrument and Equipment Development Program Chinese Academy of Science, and DNL Cooperation Fund, CAS.